Optical Biosensors for virus detection and bacteria detection is the topic of this blog article.
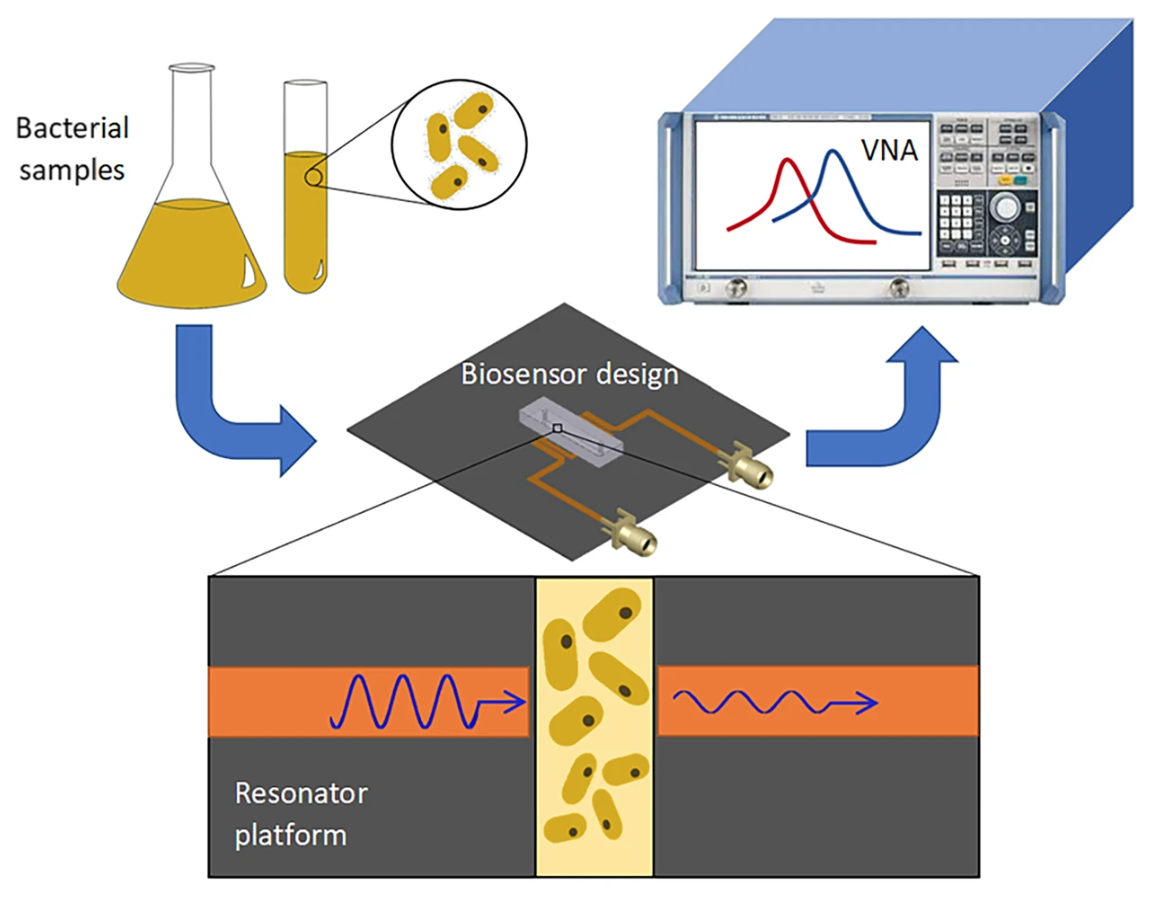
Courtesy of Nature
Introduction: The Urgent Need for Faster Diagnostics
In the modern era of global health challenges, the ability to rapidly detect viruses and bacteria has never been more critical. Traditional diagnostic tools, such as polymerase chain reaction (PCR) and enzyme-linked immunosorbent assays (ELISA), are reliable but often slow, costly, and dependent on centralized laboratories. The COVID-19 pandemic underscored the limitations of such methods, creating an urgent need for fast, accurate, and portable diagnostic solutions.
Optical biosensors have emerged as a powerful alternative. By harnessing the interaction between light and biological matter, these sensors enable label-free, real-time detection of pathogens, delivering laboratory-grade precision at the point of care.
What Are Optical Biosensors?
An optical biosensor is a device that uses a biological recognition element, such as an antibody, enzyme, or nucleic acid, coupled with an optical transducer that converts biological interactions into measurable optical signals.
The process can be summarized as:
The sensor captures target pathogens (viruses or bacteria) using a selective biological layer.
Light interacts with the bound molecules, altering optical properties (intensity, wavelength, or phase).
The optical signal is then analyzed and translated into quantitative diagnostic information.
This combination of specific biological targeting and precise photonic readout makes optical biosensors both sensitive and adaptable to a wide range of medical applications.
Key Optical Biosensing Techniques in Medical Diagnostics
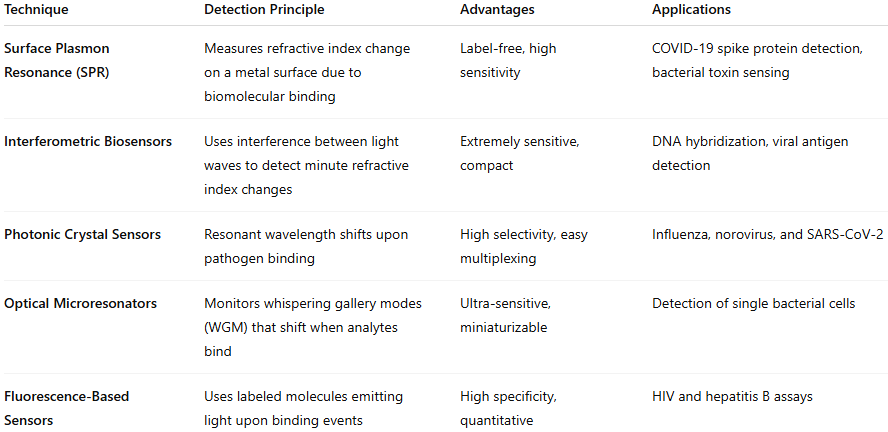
Each approach provides a unique tradeoff between sensitivity, response time, and cost. Among these, SPR and microresonator biosensors stand out for their ability to achieve real-time, label-free detection critical for rapid clinical diagnostics.
Advantages of Optical Biosensors for Virus Detection Over Traditional Methods
Conventional diagnostic methods, such as culture growth, PCR, and ELISA, remain the gold standard in many laboratories. However, they often require hours to days to yield results, trained personnel, and expensive equipment.
Optical biosensors, in contrast, offer several transformative advantages:
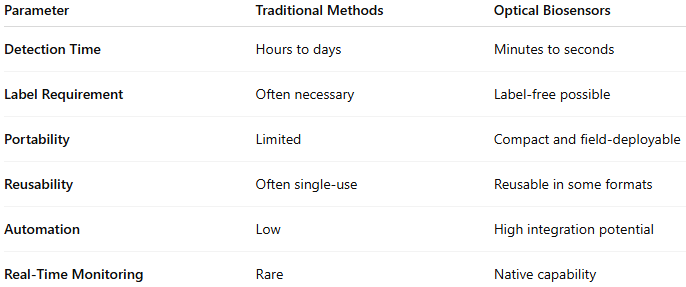
These advantages make optical biosensors ideal for rapid screening in hospitals, clinics, and emergency settings.
Photonics Enabling Next-Generation Biosensing
Photonics has become a driving force in transforming biosensing, pushing the boundaries of sensitivity, miniaturization, and real-time data acquisition. Traditional biosensing relied heavily on biochemical reactions and electrical transduction mechanisms, but photonic technologies bring unique advantages such as label-free detection, rapid response, and the ability to detect single molecules. At the core of this revolution are optical phenomena like plasmonics, interferometry, fluorescence, and Raman scattering, all harnessed through precisely engineered photonic platforms.
Integrated Photonics for Compact and Scalable Biosensors
One of the most significant developments in recent years is the rise of silicon photonics for biosensing applications. By integrating light sources, waveguides, and detectors on a single chip, these platforms enable compact and scalable systems ideal for point-of-care diagnostics. Silicon-on-insulator (SOI) waveguides, for instance, can confine light tightly within sub-micrometer dimensions, dramatically enhancing light–analyte interactions. This allows detection of biomolecular binding events in real-time with refractive index changes as small as 10⁻⁷ RIU (refractive index units).
Beyond silicon, other material platforms such as silicon nitride (Si₃N₄) and indium phosphide (InP) are gaining traction due to their low optical losses and compatibility with visible and near-infrared wavelengths commonly used in biomedical optics. Researchers at IMEC and MIT have demonstrated label-free detection of DNA hybridization and protein interactions using photonic ring resonators fabricated on Si₃N₄ chips, showing that these technologies are approaching clinical-grade performance.
Plasmonics and Nanophotonics: Enhancing Sensitivity to the Molecular Level
While integrated photonics addresses scalability, plasmonic biosensors are unparalleled in sensitivity. Surface Plasmon Resonance (SPR) and Localized Surface Plasmon Resonance (LSPR) exploit collective oscillations of electrons at metal–dielectric interfaces (typically gold or silver). These oscillations are exquisitely sensitive to refractive index changes near the surface, allowing detection of molecular binding events at femtomolar concentrations.
Nanophotonic structures such as nanohole arrays, plasmonic nanoparticles, and metasurfaces further boost sensitivity by enhancing local electromagnetic fields, known as “hot spots.” These have been integrated into lab-on-chip systems, enabling ultra-sensitive detection of biomarkers such as troponin (for early heart attack diagnosis) and specific viral antigens like SARS-CoV-2 spike proteins. In 2023, researchers at EPFL reported a metasurface-based biosensor capable of detecting single viruses in under 5 minutes using polarization-resolved spectroscopy.
Raman and Fluorescence Photonics for Multiplexed Analysis
Photonics also underpins advanced spectroscopic techniques that enable multiplexed biosensing. Raman spectroscopy, and its enhanced form Surface-Enhanced Raman Scattering (SERS), provide molecular “fingerprints” of analytes. By combining SERS with microfluidics, researchers can achieve simultaneous detection of multiple biomarkers from a single droplet of blood or saliva. Meanwhile, fluorescence-based photonic biosensors leverage controlled light–matter interactions to enhance emission signals, enabling high-throughput imaging and flow cytometry at single-cell resolution.
Emerging hybrid systems combine these optical modalities with artificial intelligence (AI) for pattern recognition and data interpretation. For example, deep-learning models trained on fluorescence and SERS spectra can now distinguish between bacterial strains or cancer cell types with over 95% accuracy, ushering in a new era of intelligent, photonic-enabled diagnostics.
Quantum and Photonic Biosensing: Pushing Beyond Classical Limits
The frontier of biosensing is extending into the quantum realm, where photon statistics and quantum correlations can surpass classical detection limits. Quantum-enhanced interferometry and entangled-photon spectroscopy promise ultra-precise measurements of absorption and refractive index changes, even under noisy conditions. Although still in early development, quantum photonic biosensing could enable the detection of molecular conformational changes and enzyme kinetics at unprecedented sensitivity.
Future Outlook: From Lab to Wearable and Point-of-Care Systems
As fabrication techniques mature, photonic biosensors are migrating from research labs to portable and wearable devices. Integration with CMOS electronics and microfluidics allows continuous monitoring of biochemical markers in sweat, tears, or breath. Companies like Rockley Photonics and Photonic Biosystems are pioneering optical sensors embedded in smartwatches capable of tracking hydration, glucose, and lactate levels in real time.
To fully realize this potential, ongoing efforts focus on improving stability, miniaturization, and multiplexing capabilities. Advances in nanofabrication, biocompatible surface coatings, and optical signal processing are key enablers. Collaborative research between academia, industry, and healthcare institutions will continue to accelerate the translation of these technologies into clinical practice.
Use Cases in Medical Diagnostics
COVID-19 Detection: SPR-based biosensors can identify SARS-CoV-2 spike proteins in under five minutes with sub-nanomolar sensitivity. Their label-free nature bypasses the need for reagents used in PCR or antigen tests.
Sepsis and Bloodborne Pathogens: Optical interferometric sensors can detect bacterial endotoxins directly in blood plasma, providing near-real-time feedback for critical patients.
Hospital-Acquired Infections: Photonic crystal sensors have shown promise in identifying methicillin-resistant Staphylococcus aureus (MRSA) from clinical samples, aiding infection control.
Tuberculosis (TB): Fluorescent biosensors using DNA probes have been developed for detecting Mycobacterium tuberculosis in sputum samples, dramatically reducing diagnostic delays.
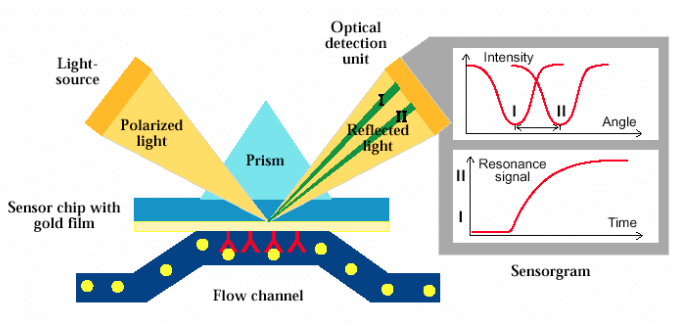
Schematic of an SPR-based biosensor (courtesy of Olivier Tillement)
These examples underscore the versatility of optical biosensors across viral, bacterial, and fungal infections—bridging lab accuracy with field usability.
Challenges and Limitations
Despite remarkable progress, several hurdles remain before optical biosensors become ubiquitous in clinical settings:
Biofouling and Sample Complexity: Real biological fluids (blood, saliva) can introduce background noise and false positives.
Standardization: Lack of unified calibration standards limits cross-lab reproducibility.
Integration with Electronics: Translating optical signals into reliable digital data for portable devices remains technically challenging.
Regulatory Approval: Biosensors must undergo stringent validation to meet FDA and ISO standards for medical diagnostics.
Overcoming these challenges will require closer collaboration between photonics engineers, biochemists, and medical device designers.
Future Outlook: The Convergence of Photonics, AI, and Healthcare
The future of optical biosensing lies in integration and intelligence. Photonic chips embedded with machine learning algorithms are already being tested for automated pattern recognition in pathogen detection. AI-driven analysis enables real-time differentiation between similar spectral signatures enhancing accuracy and speed.
Moreover, hybrid electro-optical biosensors are emerging, combining electrical readouts with optical precision to improve signal fidelity. In parallel, the rise of wearable and implantable biosensors promises continuous health monitoring detecting infections before symptoms arise.
As fabrication costs drop and optical integration scales, these biosensors are set to become as ubiquitous as glucose meters, transforming preventive medicine and public health surveillance.
Schematic view of the convergence of photonics, AI, and healthcare
Conclusion
Optical biosensors are redefining how we detect and monitor infectious diseases. By merging photonics with biotechnology, they offer rapid, accurate, and non-invasive diagnostics that are essential in today’s interconnected world.
As these devices continue to evolve integrating nanophotonics, AI, and microfluidics, they will not only complement existing laboratory tests but may soon become the frontline tools of global healthcare.
Further Reading
C. S. Zhang et al., “Optical Biosensors for Virus Detection: Advances and Prospects,” Biosensors and Bioelectronics, 2024.
M. H. Aslan et al., “Nanoplasmonic Sensors for Medical Diagnostics,” Advanced Photonics Research, 2023.
S. M. Wu et al., “Integrated Silicon Photonic Biosensors,” Nature Biomedical Engineering, 2022.
J. Homola, Surface Plasmon Resonance Based Sensors, Springer, 2020.
World Health Organization (WHO), “Innovations in Point-of-Care Diagnostics,” 2024.
