Mid-infrared spectroscopy used in disease detection with particular focus on human breath analysis is the subject of this blog post.
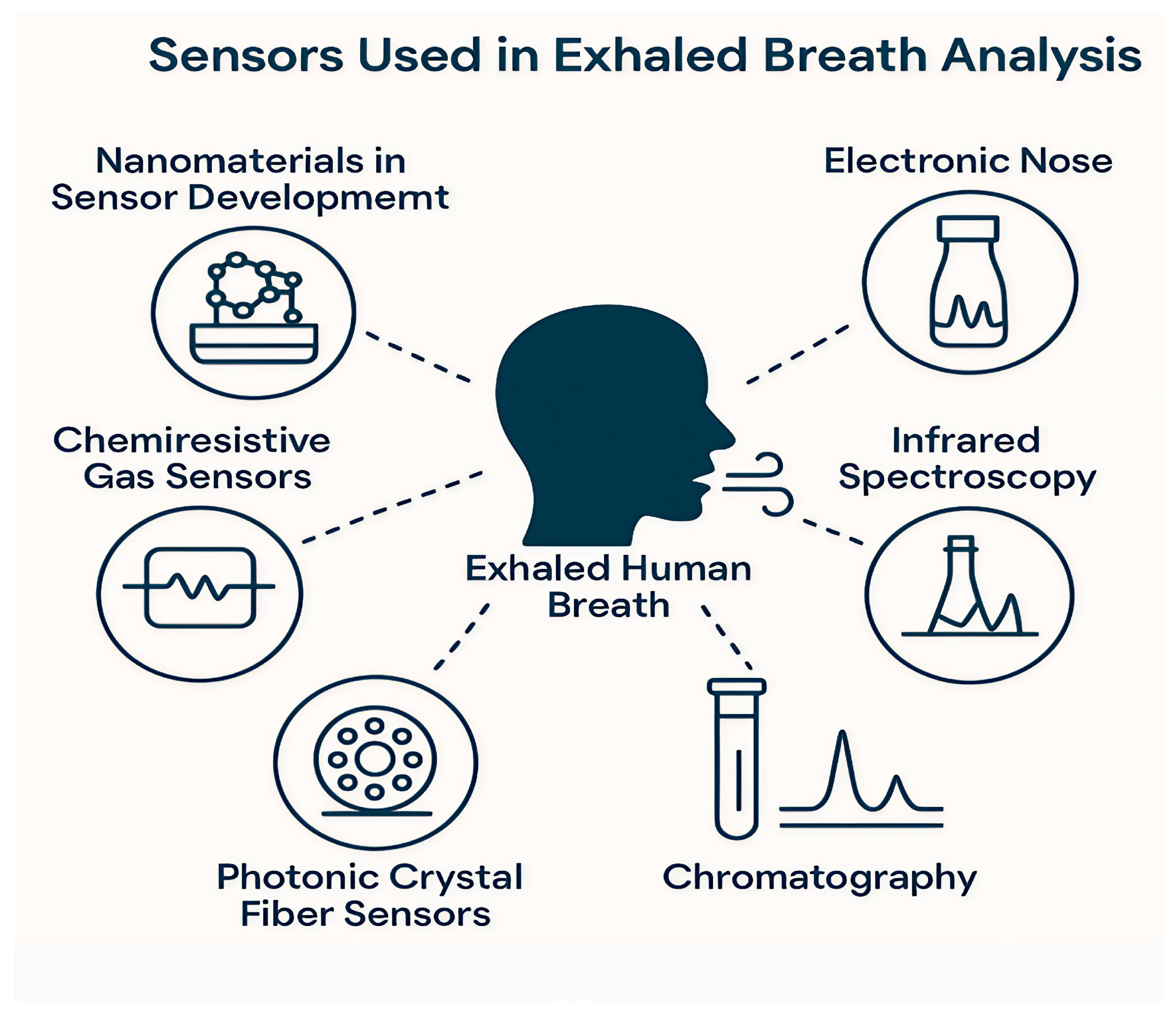
Image courtesy of S. Danishvar, et. al.
This article is brought to you by RPMC Lasers,Inc. - a trusted partner to many OEM, academic institutions and national labs for providing high-quality mid-infrared lasers and other laser solutions.
Introduction
Breath analysis is emerging as one of the most promising frontiers in non-invasive medical diagnostics. Long dominated by bulky lab instruments and labor-intensive assays, clinical testing is gradually shifting toward optical technologies that can detect the faintest biochemical fingerprints associated with disease. Among these, mid-infrared (MIR) spectroscopy stands out as an exceptionally powerful method due to its ability to probe molecular absorption bands with unmatched specificity.
Over the past decade, advances in MIR lasers, detectors, waveguides, and integrated photonics have enabled compact, highly sensitive systems capable of performing real-time, label-free analysis of exhaled breath. These systems are showing strong potential for early detection of COVID-19, lung cancer, diabetes, liver disease, and various metabolic disorders. The trend is clear: mid infrared disease detection is poised to move from the research lab to clinical environments.
In this article, we explore the technology behind MIR breath sensing, review key disease targets, and examine the engineering breakthroughs that are pushing MIR diagnostics toward mainstream adoption.
Why Breath Analysis Matters More Than Ever
Breath contains more than 800 known volatile organic compounds (VOCs), metabolic by-products, and gaseous biomarkers. Many of these compounds correlate closely with physiological processes or pathological changes occurring long before symptoms appear.
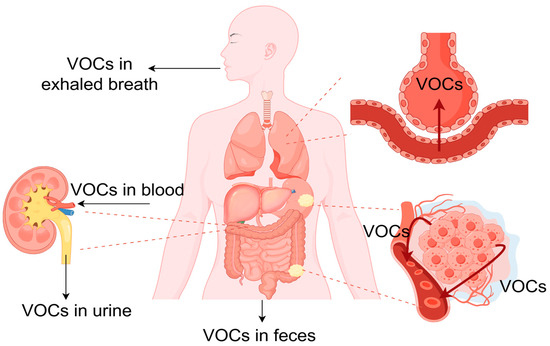
Dispersal of VOCs originating from gastrointestinal tumor tissues to various regions within the body (Courtesy of S. Danishvar, et. al.)
Traditional diagnostic tools, such as blood tests, imaging, or biopsies, are often invasive, expensive, and time-consuming. Breath analysis, on the other hand, offers:
simple, painless sample collection
rapid screening results
potential for frequent or continuous monitoring
minimal risk of contamination
What has held breath analysis back historically is sensitivity and molecular specificity. Many biomarkers appear at parts-per-million (ppm), parts-per-billion (ppb), or even parts-per-trillion (ppt) levels. MIR absorption spectroscopy directly targets the strongest fundamental vibrational modes of molecules, giving researchers a powerful mechanism to detect and quantify biomarker gases with superior precision.
The Science Behind Mid-Infrared Spectroscopy
The mid-infrared spectral region (approximately 2–20 μm) contains the fundamental molecular vibrational absorption bands for most organic molecules. This region delivers significantly stronger absorption features than those in the near-infrared, enabling detection of extremely low-concentration biomarkers.
Key Features of MIR Spectroscopy
Direct probing of molecular signatures without labels
High absorption cross-sections for VOCs
Ability to distinguish between molecules with similar structures
Compatibility with compact laser sources (QCLs, ICLs, OPOs)
The rise of Quantum Cascade Lasers (QCLs) and Interband Cascade Lasers (ICLs) has been especially transformative. These laser types provide narrow linewidths, tunability, and sufficient power for field-deployable systems.
On the detector side, advancements in HgCdTe (MCT) sensors, InAsSb, and silicon photonics-based waveguides have dramatically reduced system size and increased sensitivity.
Biomarkers and Disease Targets: What MIR Can Detect
A wide range of clinically relevant biomarkers has been identified, each associated with distinct physiological pathways. The table below summarizes key targets:
Table 1. Representative Breath Biomarkers Targeted by MIR Diagnostics
| Disease / Condition | Biomarker | MIR Detection Rationale |
|---|---|---|
| Diabetes | Acetone | Strong C=O absorption around 5.8 μm |
| Lung cancer | Formaldehyde, ethane | Sharp MIR signatures at 3.4 μm and 5.7 μm |
| Liver disease | Ammonia | Distinct N–H bands near 10 μm |
| Infectious disease (e.g., influenza) | Aldehydes & ketones | Characteristic C–H and C=O vibrations |
| Kidney dysfunction | Trimethylamine | MIR-accessible N–CH3 modes |
| Oxidative stress | Isoprene | Absorption bands around 3.3 μm |
The MIR region covers almost all key VOCs relevant to early disease detection, making it a uniquely comprehensive platform for non-invasive diagnostics.
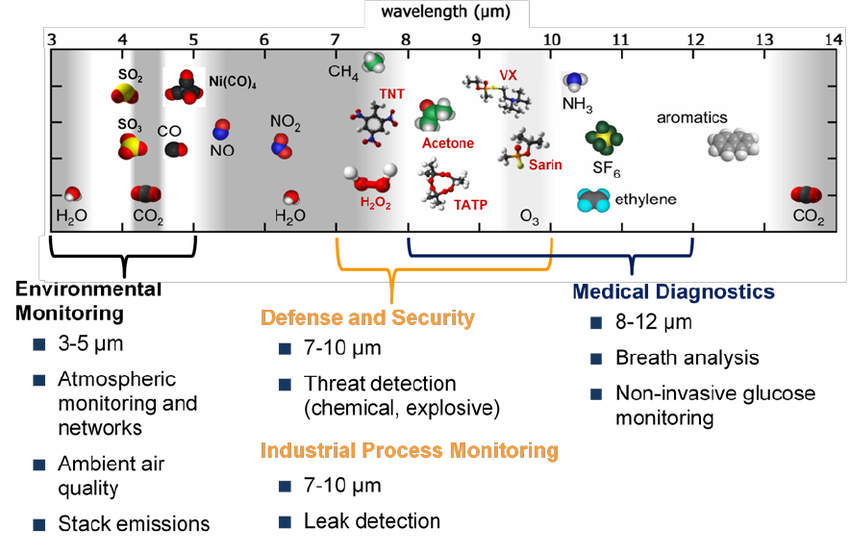
Molecules accessible in Mid-infrared spectroscopy (Courtesy of ResearchGate)
How Mid-Infrared Breath Analysis Systems Are Built
Modern MIR-based breath diagnostic systems combine multiple engineering components:
1. Tunable MIR Laser Source
Typically QCLs or ICLs operating in the 3–12 µm range, with fine tunability to sweep across absorption lines.
2. Multi-Pass Absorption Cell or Waveguide
Used to increase optical path length for enhanced sensitivity. Two common architectures:
Herriott or White cells (up to 100 m path length)
On-chip waveguides made from Si, SiGe, or chalcogenide glasses
3. Infrared Detector
Thermoelectrically cooled MCT detectors are standard, though newer photonic integrated sensors are emerging.
4. Breath Sampling Interface
Includes humidity control, flow regulation, and CO₂ correction algorithms.
5. Computational Layer
Machine learning increasingly plays a role in classifying breath signatures, compensating for humidity, and identifying biomarker combinations rather than single type of molecules.
Recent Breakthroughs Pushing MIR Diagnostics Forward
Breakthrough #1: Chip-Scale MIR Photonic Integration
Various research institutes and foundries have demonstrated MIR waveguide platforms capable of guiding wavelengths up to 11 μm. Using silicon nitride, silicon germanium, and chalcogenide glasses, researchers have built:
on-chip gas cells
frequency combs
multiplexed QCL arrays
ultra-sensitive evanescent-field sensors
These integrated systems dramatically reduce instrument size and open the door to portable medical devices.
Breakthrough #2: MIR Frequency Combs
Dual-comb spectroscopy provides extremely fast, high-resolution molecular analysis without mechanical scanning. Recent progress in QCL-based frequency combs means breath can be analyzed in milliseconds.
Breakthrough #3: AI-Powered Biomarker Classification
Deep-learning models now analyze multi-component breath spectra, identifying statistical patterns across dozens of biomarker VOCs. This multi-target approach is far more reliable than single-biomarker screening. A 2023 study from ETH Zurich showed 95% accuracy in early lung cancer detection using MIR spectroscopy combined with neural networks far surpassing traditional methods.
Breakthrough #4: MIR Sensors for Infectious Disease Screening
During the COVID-19 pandemic, MIR analyzers demonstrated the ability to distinguish infected individuals based on aldehyde profiles within seconds. Several companies are now building portable MIR pathogen-screening tools for airports and hospitals.
Clinical Applications Moving Toward Real-World Use
Clinical adoption of mid-infrared breath analysis is accelerating as hospitals, diagnostic labs, and biotech startups recognize its ability to detect molecular signatures that traditional imaging or blood tests cannot reveal early enough. One of the most compelling applications is in oncology. Tumors such as lung or colorectal cancer release distinct aldehydes, ketones, and other volatile metabolites as part of abnormal cell metabolism. These compounds can appear in exhaled breath long before a CT scan or colonoscopy shows visible anomalies. MIR spectroscopy is uniquely positioned to detect these subtle chemical fingerprints, making it a promising frontline screening method for patients with elevated risk, occupational exposures, or hereditary cancer syndromes. Several clinical trials in Europe and Asia are now benchmarking MIR-based classifiers against standard screening pathways, with early results showing strong sensitivity for early-stage disease.
Metabolic monitoring is another area where MIR technology is gaining real traction. Breath acetone is a direct biomarker of fat oxidation, and unlike glucose, insulin, or HbA1c, it responds within minutes to metabolic changes. MIR analyzers operating around 3.4 μm offer precise quantification of acetone in real time, enabling clinicians to track diabetic ketoacidosis risk, evaluate insulin resistance, or validate adherence to ketogenic or calorie-restricted diets. Biotechnology companies developing continuous metabolic monitors are now integrating quantum cascade lasers to achieve wearable or handheld MIR acetone sensors with clinical-grade accuracy.
Liver disease creates its own distinctive breath signature, primarily through elevated ammonia and a family of amine compounds. Because ammonia diffuses readily across the alveoli, its rise in exhaled breath closely mirrors blood concentration. MIR detection in the 9–11 μm region allows clinicians to monitor hepatic function without drawing blood, offering a painless alternative for patients with cirrhosis, hepatitis, or hepatic encephalopathy. Academic hospitals in Germany and Japan are currently validating MIR-based ammonia monitoring as a tool for rapid triage in emergency rooms.
Inflammatory conditions also alter the composition of VOCs, generating nitriles, hydrocarbons, and oxidized molecules linked to oxidative stress. MIR analyzers can quantify these chemical shifts with high specificity. This capability opens the door to rapid, bedside detection of acute inflammation, including sepsis, asthma exacerbation, and chronic obstructive pulmonary disease flare-ups, where standard diagnostic workflows often require blood panels and lengthy processing times.
Interest has surged as well in infectious disease screening. Viral and bacterial infections disrupt metabolic pathways, producing unique patterns of breath biomarkers. Studies performed during and after the COVID-19 pandemic demonstrated that MIR detection of elevated nitric oxide, specific VOC clusters, and exogenous compounds could provide rapid, contact-free triage. Because MIR systems can be made compact and require no reagents, they are now being evaluated for point-of-entry health screening at airports, hospitals, and nursing facilities. Emerging prototypes using integrated photonic MIR chips show analysis times under 30 seconds and accuracy levels comparable to PCR-validated breath studies for certain respiratory pathogens.
Together, these clinical pathways illustrate a clear trend: mid-infrared spectroscopy is no longer limited to research laboratories. It is evolving into a practical, patient-friendly diagnostic platform capable of addressing screening, monitoring, and rapid triage across multiple medical specialties. As MIR systems become smaller, more affordable, and more integrated with cloud-based analysis, their role in routine clinical practice is expected to expand rapidly.
Challenges That Must Still Be Solved
Despite remarkable progress, several hurdles remain:
Humidity Interference
Water vapor is one of the strongest absorbers in the mid-infrared region. Its rotational–vibrational bands span large portions of the 2–12 μm spectrum, which makes humidity the single largest environmental barrier to accurate MIR breath analysis. Even small fluctuations in ambient moisture levels can distort baselines, obscure biomarker signatures, and reduce quantification accuracy for low-concentration VOCs. This is especially problematic for point-of-care devices used outside of controlled laboratory environments.
To manage this, modern MIR breath analyzers are built with multi-layered humidity mitigation strategies. One approach is real-time spectral correction using advanced algorithms such as multi-component fitting, baseline subtraction driven by water vapor line databases (e.g., HITRAN), and machine-learning regression models that isolate interfering H₂O features from target molecules. These computational tools can compensate for humidity variations without requiring heavy hardware preprocessing.
Standardization of Biomarker Thresholds
A major challenge in translating mid-infrared breath analysis into routine clinical practice is the lack of universally accepted biomarker thresholds. Research groups often report different concentration ranges for the same disease-related VOCs, and these discrepancies frequently stem from inconsistent sampling and processing methods rather than biological variability. To address this, the breath analysis community is moving toward standardized protocols. The International Association for Breath Research (IABR) and several EU research consortia (e.g., FABULOUS, SniffPhone) have proposed harmonized guidelines covering breath sampling, moisture control, storage, calibration gases, and metadata reporting. Some groups are also developing validated reference materials, controlled VOC mixtures spanning clinically relevant concentration ranges, to allow instrument-to-instrument comparison.
Regulatory Pathways
For MIR devices to reach clinics, they must undergo rigorous medical validation and regulatory approval.
Miniaturization
While chip-scale components exist, fully integrated MIR breath analyzers remain a work in progress.
Looking Ahead: The Future of MIR Breath Diagnostics
As MIR components continue to shrink and AI-based spectral analysis improves, breath analysis could become a routine part of medical examinations — as common as a blood pressure check.
The next decade may see:
compact MIR breath analyzers in primary-care clinics
home monitoring systems for chronic disease
airport disease-screening portals
integration of MIR sensors into smartphones or wearables
The convergence of photonic integration, materials science, and AI is positioning mid infrared disease detection as one of the defining diagnostic technologies of the future.
